When talking with people about survival situations or self-reliance skills, you may have heard the topic of distillation come up.
Many people associate the term distillation with the production of alcohol, which it certainly is a part of. But it has other applications as well. Let’s start with a simple definition of this term.
The Merriam Webster Dictionary defines distillation as “the process of purifying a liquid through evaporation and condensation.”
Pretty simple, right? No? Okay, let me break this down into a simple step-by-step process.
Breakdown of Water Distillation
According to the above definition we want to purify a liquid which means we want to separate the liquid from other liquids or substances.
So for example, I’m going to talk about a mixture of water and alcohol.
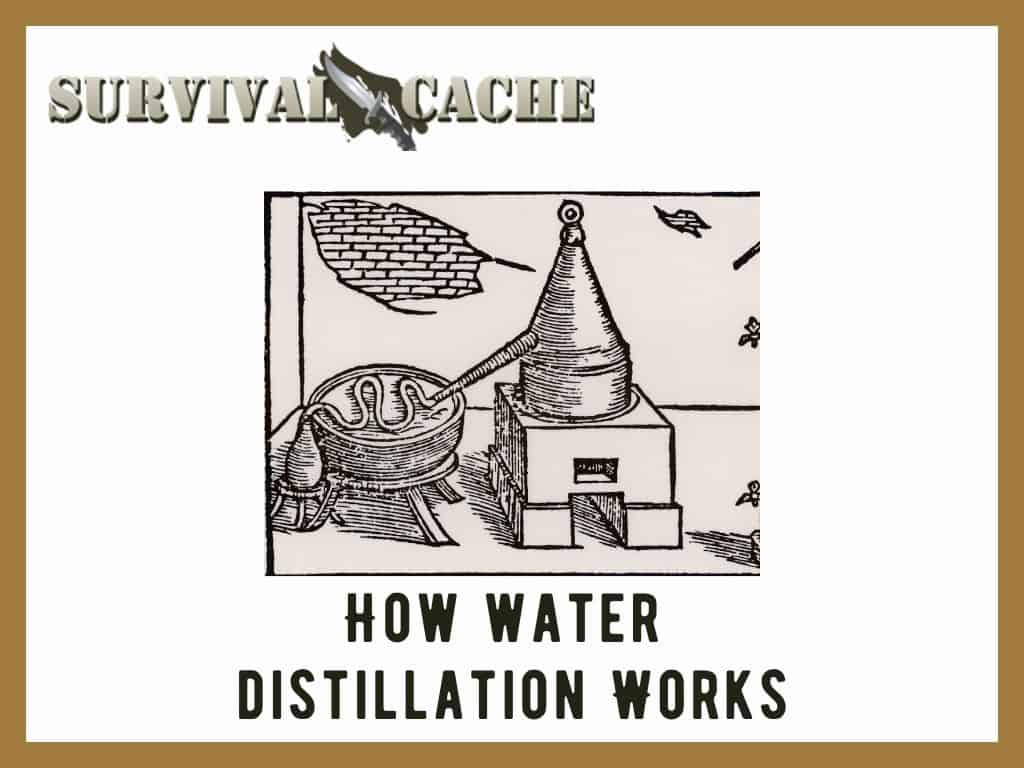
Now, the first thing that is needed is an apparatus for conducting this experiment.
In its most simple form, a distiller is made up of five components.
A container to boil the mixture in, a heat source for boiling the mixture, a tube for transferring the evaporated liquid, a cooling source for condensing the liquid, and a collection container.
In my example, the water and alcohol mixture are put into the first container and then heated. Water has a much higher boiling point than alcohol so the alcohol will evaporate first.
As the mixture is heated the alcohol will turn into a gas (evaporation) and rise upward. It will then be forced into the transfer tube.
Here it is cooled by either air or more commonly cold water that surrounds the transfer tube. When it cools, it condenses back into a liquid. The liquid then flows into the collection container.
During the process, some water may be transferred over with the alcohol which is why the process may be repeated several times. When the process is repeated more of the alcohol is separated from the water and a higher yield of alcohol is achieved.
Distillation and Survival Situations
Other than being consumed, alcohol has its place in aseptic techniques. But in terms of survival situations, distillation is more important in creating potable water.
The most popular example is turning saltwater into freshwater. This process is referred to as desalination and is almost the same as described above with one small difference.
A mixture of saltwater is placed into a container and then heated. As the water is heated it turns into a gas (evaporates) and travels upward while leaving the salt behind in the container.
The gaseous water travels through the transfer tube, is cooled, and is collected on the other side. As simple as this sounds, in a survival situation, the materials may be difficult to come by to properly construct a desalination unit.
But you do not have to wait for such a situation to arise to make one of these because they can be useful even if you are not stranded on a deserted island surrounded by saltwater.
How to make a Simple Desalination Unit
To show you how simple it is to make one of these I decided to not order or buy any special materials. Instead, I will be making one out of materials that I found around my home.
Boiling Container
The first item that I needed was a container to boil a mixture of water in. since the container is going to be subjected to high temperatures a container made from plastic is out of the question.
I decided on a glass jar with a lid. Some thinner-walled glass bottles may not be appropriate to use as they could shatter while being heated up.
Now, in a true survival situation, a plastic container can be used to boil water at low temperatures. But as the plastic is heated, harmful chemicals can leach into the water supply. In such a situation it is up to you to decide if circumstances are dire enough to drink from such a source.
Transfer Tube
Now on to the transfer tube. I found a small length of copper tubing in the garage that should work well for this purpose. Small copper tubing is common if one knows where to look.
Again, I would recommend staying away from general use plastic tubing for this purpose. The steam that is entering the transfer tube is quite hot and may cause the tubing to leach.
Collection Container
As far as the collection container goes, almost anything can be used. But, ideally, the collection container will be sealed around the copper tubing to prevent any steam that did not condense, from escaping.
I decided to use another glass bottle with a screw-on lid. I then poked a hole in both lids that were just a little bit smaller than the copper tube. When I pushed the tube through the hole, the lid sealed decently around the tube.
Cooling Method
The last thing to consider is the cooling method around the copper tubing. Now I have done this before and allowing the tube to cool by air does work, just not efficiently. Cooling by air may produce a mixture of steam and liquid water.
“Contaminated water” can be used to cool the tube by simply wrapping a wet rag around the tubbing. However, the rag will have to be wetted numerous times throughout the process because the tubing will heat up over time.
When using untreated water to cool the transfer tube be sure that none of it gets into the clean water collection container.
Testing it Out
For demonstration purposes, I decided to do this inside on the stove top using a double boiler method.
To start, I placed a fair amount of salt into the glass jar. I did not measure the amount of salt, but it is more than enough to turn the water into a milky white color.
Next, I connected the tubing, set everything where I wanted it to go, and began heating.
I decided to opt-out of cooling the tube with water and instead wanted to see how well air cooling would work.
It took about twenty minutes before I started to see any moisture coming out of the tube on the clean water side.
After about forty-five minutes I decided to stop and make some adjustments because the results were about one teaspoon of clean water.
A Few Adjustments
The seal around the copper tubing and the lid were not as tight as I thought and I was losing quite a bit of steam on the heated bottle side.
So, I took a small piece of a washcloth and shoved it around the lid and copper tubing.
I also decided to put the jar directly onto the heat source instead of using the double boiler. I DO NOT RECOMMEND DOING THIS.
With a glass jar not designed for this purpose, this is not a good idea because the jar could shatter. But I took the chance for the sake of the article.
After the Adjustments
The contaminated water heated up much quicker and was soon boiling in the jar.
With the piece of a washcloth in place, there was zero steam escaping from around the tube. This meant all the steam was now being forced into the copper tube.
Within about five minutes of boiling, there was a more consistent stream of drips into the clean container.
But it still took about thirty minutes before I saw roughly a few tablespoons of clean water.
Making it Better
So this project I wanted to do as simply as possible just to show the process and that it can be done.
Next time however I will be stepping up my game and making a much more efficient unit. These will be the three things I will be fixing.
- The boiling container
- Adding seals
- Adding a water cooler
My next attempt will include a container that can be applied directly to a heat source, it will be properly sealed, and it will be water-cooled.
Water does a much better job of cooling down the transfer tube and speeding up the condensation process.
Alternative Methods of Water Distillation
But what about if you do not have the means to make a fire or the materials to make a desalination unit as described above?
Well, in that case, I did want to offer two additional methods for turning saltwater into freshwater.
Both methods are considered solar stills, but their designs are slightly different.
Method 1
The first method is pretty well known because it has been brought up a lot within the outdoor community.
The only special materials that are required are a sheet of clear plastic and a collection container.
Start by digging a hole a few feet wide by a few feet deep. Line the hole with green vegetation or pour in saltwater.
Next, place the collection container in the center of the hole and then cover the hole with a sheet of plastic.
Place a small pebble on the center of the plastic so that the material dips down just over the collection container.
As the hole heats up the saltwater will evaporate and water droplets will begin to collect on the underside of the plastic.
Eventually, it will run down to the center of the plastic and drip into the collection container.
Method 2
This method is less work than the previous one but it will require three containers. Each container needs to be smaller than the previous one and you will see why in a second.
For demonstration purposes, let’s use a small pot, a 2-liter plastic bottle, and an old soup can.
Fill the soup can with salt water and place it in the center of the pot. Then cut the bottom off the 2-liter bottle and place it over the soup can.
Place the whole setup in direct sunlight. As the water in the soup can heat up it will evaporate and become trapped by the 2-liter bottle. The condensation will collect at the top of the bottle and run down the side where it will be collected in the pot.
Wrap Up
With the right materials and a little prep time, a desalination unit can easily be constructed. This simple system gives a person the ability to take saltwater and create drinkable freshwater.
Thanks for reading and stay prepared!
Have you ever made a desalination unit? Sound off in the comment section below and lets us know about your experience.
https://ift.tt/3lRrnvH
via Survival Cache https://ift.tt/2JVMyw1
March 25, 2021 at 11:48AM

0 Comments